How the Enthesis changed our understanding of the Immune System
Introduction
From the dawn of the modern Immunology era in the late 19th century it was thought that most non-infectious inflammatory diseases were "autoimmune".
Autoimmunity can be defined as a situation where the adaptive immune system drives the inflammation by virtue of its ability to recognise different tissue constituents and its ability to show immunological memory.
The cells responsible for this are B and T lymphocytes.
Consequently an attack arising in the lymphoid glands was thought to the principal mechanism that lead to the destruction of previously normal tissues.
In the late 1990s studies showed that genetic abnormalities in cells arising outside the lymph nodes were capable of causing tissue damage.
This was dubbed "autoinflammation.
How Enthesitis changed the understanding of immunity
Work by the Leeds-Cardiff group showed that the normal enthesis was a site of microdamage and microscopic inflammation.
The enthesis is a site of high mechanical stress in Psoriatic Arthritis.
Psoriasis can also be triggered by stress or injury to the skin.
Likewise nail disease in psoriasis is commoner over the dominant finger nails which is likely to be due to physical stress.
This leads to the realisation that "tissue specific factors" at the enthesis and not the immune system might drive inflammatory disease in the pattern of disease distribution in the human Spondyloarthropathies.
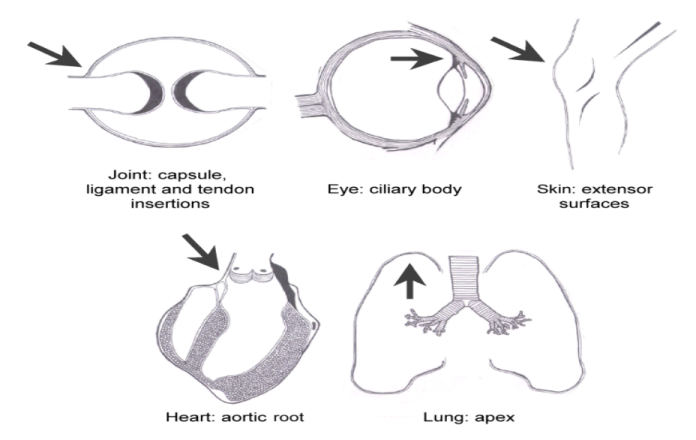 |
McGonagle et al J Rheum 2001 |
Consequently we offered a new definition for autoinflammation that had been poorly defined until that time.
 |
Autoinflammation - Generic Definition Self directed inflammation, whereby local tissue factors lead to activation of innate immune responses. These responses play the dominant role, with disturbed homeostasis of canonical cytokine cascades or related molecules, defective bacterial sensing and other local factors leading to disease expression. |
This allowed us to set a new boundary for inflammation against self.
From this we developed the immunological Disease Continuum Classification of arthritis.
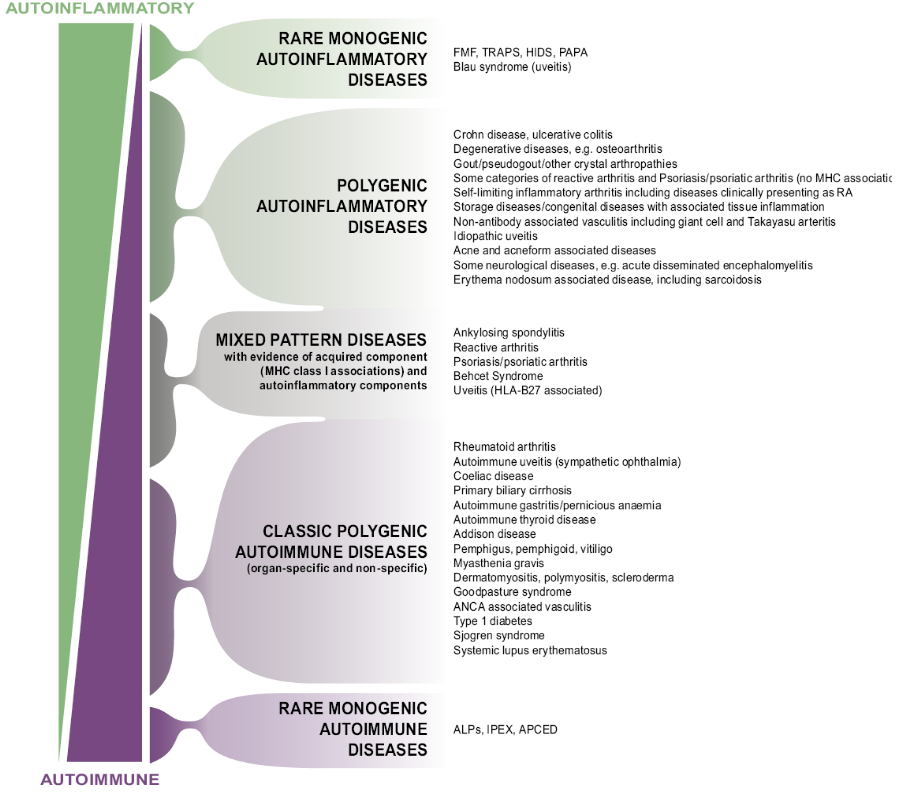 |
McGonagle & McDermott PLoS Med 2006 |
The enthesitis related conditions or the Spondyloarthropathies set near the top of this scheme.
The diffuse pattern of pathology around insertional points in Psoriatic Arthritis attests to the role of a tissue specific factor, namely mechanical stressing of joints, as a driving force for disease (Figure 4).
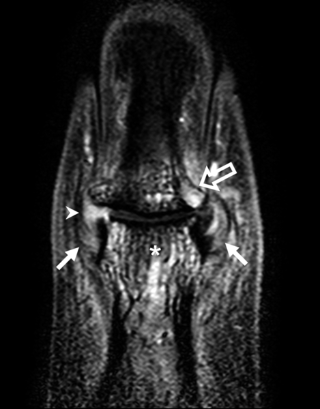 |
Fig 4. Recognition of Autoinflammation: Psoriatic Arthritis as an Example In early RA, joint disease localisation is to the synovium in keeping with the concept of the synovium being the primary target organ. However, in early psoriatic arthritis, the inflammatory changes have a widespread distribution and appear to relate to patterns of joint stressing around ligaments, adjacent bone, and soft tissues, rather than a specific antigen territory. The figure shows a contrast-enhanced high-resolution magnetic resonance image of a distal interphalangeal joint optimised for showing sites of inflammation (pixel size, 100 x 100 microns). There are extensive inflammatory changes in all tissues. Asterisk, site of diffuse osteitis; arrowhead, synovial enhancement; solid arrows, joint ligaments that show florid inflammatory changes at insertions and within ligaments; open arrow, extracapsular soft-tissue enhancement. |
Whilst initially appearing to be counter intuitive to mainstream immunology these concepts have now been proven to hold true in animal models.
Firstly, proinflammatory chemicals called cytokines can trigger inflammation at entheses [1].
Secondly novel innate immune cells at the enthesis can also drive disease [2].
Thus from being a fairly neglected structure in medicine and biology the enthesis has profoundly changed the way the immune system is viewed [2].
References

